Clinical trial solution using AI-based ‘digital twins’ – Attracted $12 million investment
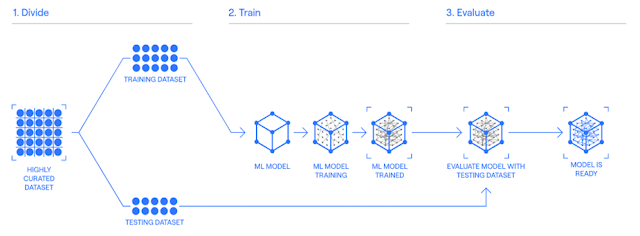
Reduce the number of subjects and time required for clinical trials by making it possible to predict the placebo effect when administering a placebo with the same 'digital twin' as the subject.
‘Digital twins’ are modeled based on tens of thousands of placebo effect-related subject data and can be created in just one subject visit.
Unlearn.AI
Unlearn.AI is a developer of a platform designed to make computational clinical trials. The company's platform accelerates clinical trials by supplementing control groups with synthetic patient data generated using AI, that helps in reducing the time to develop new medicines, enabling healthcare companies to sooner provide patients in need with life-saving therapies.
General Information
- Headquarters : San Francisco, CA
- Founders : Charles Fisher
- Categories : Artificial Intelligence, Bioinformatics, Biopharma, Clinical Trials, Computer, Life Science, Machine Learning, Software
- Founded : 2017
- Contact : info@unlearn.ai
Funding
- Investors: 8VC, Data Collective DCVC, Mubadala Capital | Ventures US
- Funding Rounds (1) - $12M
- Apr 20, 2020 Series A / $12M
Source: Unlearn.AI
👉• Web: https://www.unlearn.ai
👉• Video:












0 Comments